is aldehyde dangerous In defense of aldehydes
When it comes to chemistry, there are various properties, structures, uses, and examples of different chemicals. One such chemical is acetaldehyde, which is widely used in the industrial and chemical sectors. In this post, we will discuss the various properties of acetaldehyde along with its structure, uses, and examples.
Structure of Acetaldehyde
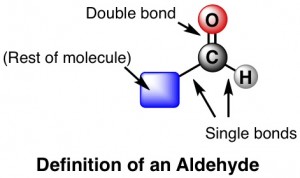
Acetaldehyde is a colorless, flammable liquid that is widely used in the chemical industry. It has a molecular formula of C2H4O and a molar mass of 44.05 g mol-1. The chemical structure of acetaldehyde consists of a methyl group (-CH3) and a carbonyl group (-CHO) attached to a single carbon atom. The carbonyl group is responsible for the aldehyde functional group, which is the main characteristic of the chemical.
 Properties of Acetaldehyde
Properties of Acetaldehyde
Acetaldehyde has various physical and chemical properties that make it useful in different fields. Some of its properties are:
- Boiling point: 20.2 °C
- Melting point: -123.5 °C
- Density: 0.78 g cm-3
- Solubility in water: miscible
- Odor: pungent and fruity
Acetaldehyde is highly reactive due to the presence of the carbonyl group, which makes it an important intermediate in various chemical reactions. It can undergo different reactions such as oxidation, reduction, and condensation, among others.
Uses of Acetaldehyde
Acetaldehyde is widely used in the chemical and industrial sectors due to its various properties. Some of its uses are:
- Production of acetic acid: Acetaldehyde is used in the production of acetic acid, which is used in the food industry as a preservative and flavoring agent.
- Production of resins and plastics: Acetaldehyde is used in the production of resins and plastics such as polyvinyl acetate.
- Production of pharmaceuticals: Acetaldehyde is used in the production of various pharmaceuticals such as vitamin B1.
- Production of perfumes: Acetaldehyde is used in the production of perfumes and fragrances due to its fruity odor.
 Examples of Acetaldehyde
Examples of Acetaldehyde
There are various examples of acetaldehyde that can be found in everyday life. Some of these are:
- Ripe fruits such as grapes, apples, and bananas contain acetaldehyde which gives them their characteristic odor and flavor.
- Vegetables such as tomatoes and spinach contain acetaldehyde which plays a key role in their ripening process.
- Alcoholic beverages such as beer and wine contain acetaldehyde which is produced during their fermentation process.
- Cigarette smoke contains acetaldehyde which is harmful to health and can cause various diseases such as cancer, pulmonary fibrosis, and asthma.
In conclusion, acetaldehyde is a highly reactive chemical with various properties, structures, uses, and examples. It is widely used in the chemical and industrial sectors and can be found in everyday life in various forms. Understanding the various aspects of acetaldehyde is important in order to ensure its safe and efficient use in different fields.
If you are looking for In Defense of Aldehydes | I Can Has Science? you’ve visit to the right web. We have 5 Pictures about In Defense of Aldehydes | I Can Has Science? like الفرق بين الألدهيد والكيتون - أخبار 2023, In Defense of Aldehydes | I Can Has Science? and also الفرق بين الألدهيد والكيتون - أخبار 2023. Read more:
In Defense Of Aldehydes | I Can Has Science?
 icanhasscience.comaldehydes
icanhasscience.comaldehydes
الفرق بين الألدهيد والكيتون - أخبار 2023
 ar.weblogographic.comThe Product(s) Is / Are: | Chemistry Questions
ar.weblogographic.comThe Product(s) Is / Are: | Chemistry Questions
 www.toppr.comAcetaldehyde - Properties, Structure, Uses And Examples - Infinity Learn
www.toppr.comAcetaldehyde - Properties, Structure, Uses And Examples - Infinity Learn
 infinitylearn.comNews | Fanconi Anemia Research Fund
infinitylearn.comNews | Fanconi Anemia Research Fund
 www.fanconi.orgaldehydes reactive fanconi damage orphan longer dna potent source these
www.fanconi.orgaldehydes reactive fanconi damage orphan longer dna potent source these
The product(s) is / are:. Aldehydes reactive fanconi damage orphan longer dna potent source these. In defense of aldehydes